Viruses can provide answers to questions we have never even
asked.
A
virus is a parasite.
Viruses reproduce rapidly and often with violent results, yet they are so
rudimentary that many scientists don’t even consider them to be alive. A
virus is nothing more than a few strands of genetic material wrapped in a
package of protein—a parasite, unable to function on its own. In order
to survive, it must find a cell to infect. Only then can any virus make
use of its single talent, which is to take control of a host’s cellular
machinery and use it to churn out thousands of copies of itself. These
viruses then move from one cell to the next, transforming each new host
into a factory that makes even more virus. In this way, one infected cell
soon becomes billions.
Viruses cause plagues.
Nothing—not even the Plague—has posed a more persistent threat to
humanity than viral diseases: yellow fever, measles, and smallpox have
been causing epidemics for thousands of years. At the end of the First
World War, fifty million people died of the Spanish flu; smallpox may have
killed half a billion during the twentieth century alone. Those viruses
were highly infectious, yet their impact was limited by their ferocity: a
virus may destroy an entire culture, but if we die it dies, too. As a
result, not even smallpox possessed the evolutionary power to influence
humans as a species—to alter our genetic structure. That would require
an organism to insinuate itself into the critical cells we need in order
to reproduce: our germ cells. Only retroviruses, which reverse the usual
flow of genetic code from DNA to RNA, are capable of that. A retrovirus
stores its genetic information in a single-stranded molecule of RNA,
instead of the more common double-stranded DNA.
Viruses sometimes get into an egg or into the sperm.
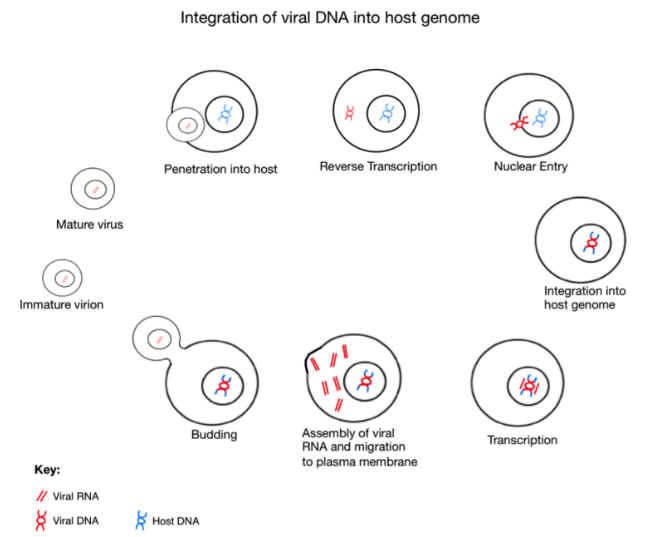
When it infects a cell, the virus deploys a special enzyme, called reverse
transcriptase, that enables it to copy itself and then paste its own genes
into the new cell’s DNA. It then becomes part of that cell forever; when
the cell divides, the virus goes with it. Scientists have long suspected
that if a retrovirus happens to infect a human sperm cell or egg, which is
rare, and if that embryo survives—which is rarer still—the retrovirus
could take its place in the blueprint of our species, passed from mother
to child, and from one generation to the next, much like a gene for eye
color or asthma.
Lots of left over DNA.
When the sequence of the human genome was fully mapped, in 2003,
researchers also discovered something they had not anticipated: our bodies
are littered with the shards of such retroviruses, fragments of the
chemical code from which all genetic material is made. It takes less than
two per cent of our genome to create all the proteins necessary for us to
live. Eight per cent, however, is composed of broken and disabled
retroviruses, which, millions of years ago, managed to embed themselves in
the DNA of our ancestors. They are called endogenous retroviruses, because
once they infect the DNA of a species they become part of that species.
One by one, though, after molecular battles that raged for thousands of
generations, they have been defeated by evolution. Like dinosaur bones,
these viral fragments are fossils. Instead of having been buried in sand,
they reside within each of us, carrying a record that goes back millions
of years. Because most no longer seem to serve a purpose or cause harm,
these remnants have often been referred to as “junk DNA.” Many still
manage to generate proteins, but scientists have never found many that
function properly in humans or that could make us sick.
Resurrecting a virus.
Then, in 2003, Thierry Heidmann brought one back to life. Combining the
tools of
genomics, virology, and evolutionary biology, he and his colleagues took a
virus that had
been extinct for hundreds of thousands of years, figured out how the
broken parts were
originally aligned, and then pieced them together. After resurrecting the
virus, the team
placed it in human cells and found that their creation did indeed insert
itself into the DNA
of those cells. They also mixed the virus with cells taken from hamsters
and cats. It quickly
infected them all, offering the first evidence that the broken parts could
once again be made
infectious. The experiment could provide vital clues about how viruses
like H.I.V. work.
Inevitably, though, it also conjures images of Frankenstein’s monster
and Jurassic Park.
“If you think about this for five minutes, it is wild stuff,” John
Coffin said in his laboratory at Tufts University, where the American
Cancer Society Research is.
Professor Coffin is one of the country’s most distinguished molecular
biologists, and was
one of the first to explore the role of endogenous retroviruses in human
evolution. “I
understand that the idea of bringing something dead back to life is
fundamentally
frightening, It’s a power that science has come to possess and it makes
us
queasy, and it should. But there are many viruses that are more dangerous
than these—more infectious, far riskier to work with, and less
potentially useful.’’
Building a polio virus.
Thanks to steady advances in computing power and DNA technology, a
talented
undergraduate with a decent laptop and access to any university biology
lab can assemble a
virus with ease. In 2002, as if to prove that point, researchers from the
State University
of New York at Stony Brook “built” a polio virus, using widely
available information and
DNA they bought through the mail. To test their “polio recipe,” they
injected the virus into
mice. The animals first became paralyzed and then died. (“The reason we
did it was to prove
that it can be done,’’ Eckard Wimmer, who led the team, said at the
time. “Progress in
biomedical research has its benefits and it has its downside.’’) The
effort was widely seen as pointless and the justification absurd. “Proof
of principle for bioterrorism,’’ Coffin called it.
“Nothing more.” Then, two years ago, after researchers had sequenced
the genetic code of
the 1918 flu virus, federal scientists reconstructed it, too. In that
case, there was a well understood and highly desired goal: to develop a
vaccine that might offer protection against
future pandemics.
Phoenix virus.
Resurrecting an extinct virus is another matter. Still, if Heidmann had
stuck to scientific
nomenclature when he published his results, few outside his profession
would have
noticed. A paper entitled “Identification of an Infectious Progenitor
for the Multiple-Copy
HERV-K Human Endogenous Retroelements,’’ which appeared in the journal
Genome
Research, was unlikely to cause a stir. Heidmann is on a bit of a mission,
though. He named
the virus Phoenix, after the mythical bird that rises from the ashes,
because he is convinced
that this virus and others like it have much to tell about the origins and
the evolution of
humanity.
ERV's determined how we evolved.
With equal ardor but less fanfare, scientists throughout the world have
embarked on similar
or related projects. One team, at the Aaron Diamond AIDS Research Center,
in New York,
created an almost identical virus. In the past, groups at Oxford
University and at the Fred Hutchinson Cancer Research Center, in Seattle,
have also produced results that provide startling observations about
evolution and disease. The approaches often differ, but not the goals. All
of these researchers hope that excavating the molecular past will help
address the medical complexities that we confront today. Almost
incidentally, they have created a new discipline, paleovirology, which
seeks to better understand the impact of modern diseases by studying the
genetic history of ancient viruses.
"This is something not to fear but to celebrate,’’ Heidmann said.
His office at the
institute is dedicated to the treatment and eradication of cancer.
“What is remarkable here, and unique, is the fact that endogenous
retroviruses are two things at once:
genes and viruses. And those viruses helped make us who we are today just
as surely as other genes did. I am not certain that we would have survived
as a species without them.
The Phoenix virus sheds light on how H.I.V. operates, but, more than that,
on how we operate, and how we evolved. Many people study other aspects of
human
evolution—how we came to walk, or the meaning of domesticated animals.
Equally important is the role of pathogens in shaping the way we are
today. Look,
for instance, at the process of pregnancy and birth."
Placenta evolution...and AIDS.
Without endogenous retroviruses mammals might never have developed the
placenta,
which protects the fetus and gives it time to mature. That led to live
birth, one of the
hallmarks of our evolutionary success over birds, reptiles, and fish. Eggs
cannot eliminate
waste or draw the maternal nutrients required to develop the large brains
that have made
mammals so versatile. These viruses made those changes possible, It
is quite possible that, without them, human beings would still be laying
eggs.
H.I.V., the only retrovirus that most people have heard of, has caused
more
than twenty-five million deaths and infected at least twice that number of
people since the middle of the twentieth century, when it moved from
monkey to man. It may be hard to understand how organisms from that same
family, and constructed with the same genes, could have played a
beneficial, and possibly even essential, role in the health and
development of any species. In 1968, Robin Weiss, who is now a professor
of viral oncology
at University College London, found endogenous retroviruses in the embryos
of healthy
chickens. When he suggested that they were not only benign but might
actually perform a
critical function in placental development, molecular biologists laughed.
“When I first
submitted my results on a novel ‘endogenous’ envelope, suggesting the
existence of an
integrated retrovirus in normal embryo cells, the manuscript was roundly
rejected,’’ Weiss
wrote last year in the journal Retrovirology. “One reviewer pronounced
that my
interpretation was impossible.’’
Descent from viruses.
Weiss, who is responsible for much of the basic knowledge about how
the AIDS virus interacts with the human immune system, was not deterred.
He
was eager to learn whether the chicken retroviruses he had seen were
recently acquired
infections or inheritances that had been passed down through the
centuries. He moved to
the Pahang jungle of Malaysia and began living with a group of Orang Asli
tribesmen. Red
jungle fowl, an ancestor species of chickens, were plentiful there, and
the tribe was skilled at
trapping them. After collecting and testing both eggs and blood samples,
Weiss was able to
identify versions of the same viruses. Similar tests were soon carried out
on other animals.
The discovery helped mark the beginning of a new approach to biology.
“If Charles Darwin
reappeared today, he might be surprised to learn that humans are descended
from viruses as well as from apes,” Weiss wrote.
Evidence of evolution.
Darwin’s surprise almost certainly would be mixed with delight: when he
suggested, in “The
Descent of Man” (1871), that humans and apes shared a common ancestor,
it was a
revolutionary idea, and it remains one today. Yet nothing provides more
convincing evidence
for the theory of evolution than the viruses contained within our DNA.
Until recently, the
earliest available information about the history and the course of human
diseases, like
smallpox and typhus, came from mummies no more than four thousand years
old. Evolution
cannot be measured in a time span that short. Endogenous retroviruses
provide a trail of
molecular bread crumbs leading millions of years into the past.
Proof of common descent.
Darwin’s theory makes sense, though, only if humans share most of those
viral fragments
with relatives like chimpanzees and monkeys. And we do..in thousands of
places throughout our genome. If that were a coincidence, humans and
chimpanzees would have had to endure an incalculable number of identical
viral infections in the course of millions of years, and then, somehow,
those infections would have had to end up in exactly the same place within
each genome. The rungs of the ladder of human DNA consist of three billion
pairs of
nucleotides spread across forty-six chromosomes. The sequences of those
nucleotides
determine how each person differs from another, and from all other living
things. The only
way that humans, in thousands of seemingly random locations, could possess
the exact
retroviral DNA found in another species is by inheriting it from a common
ancestor.
Molecular biology has made precise knowledge about the nature of that
inheritance possible.
With extensive databases of genetic sequences, reconstructing ancestral
genomes has become common, and retroviruses have been found in the genome
of every vertebrate species that has been studied.
Other animals.
Anthropologists and biologists have used them to investigate not only the
lineage of primates but the relationships among animals—dogs, jackals,
wolves, and foxes,
for example—and also to test whether similar organisms may in fact be
unrelated.
Although it is no longer a daunting technical task to find such viruses,
or their genes,
figuring out the selective evolutionary pressures that shaped them remains
difficult. Partly,
that is because the viruses mutate with such speed.
H.I.V.
H.I.V. can evolve a million times as fast as the human-immune-system cells
it infects. (Such constant change makes it hard to develop antiviral drugs
that will remain effective for long, and it has also presented a
significant obstacle to the development of an AIDS vaccine.)
There are retroviruses (like H.I.V.) that do not infect sperm or egg
cells. Because they are
not inherited, they leave no trace of their history. “We can have a
fossil record only of the
viruses that made it into the germ line,’’ Paul Bieniasz said. “And,
of course, most did
not.” Bieniasz is a professor of retrovirology at the Aaron Diamond AIDS
Research Center
and the chief of the retrovirology laboratory at Rockefeller University.
He has long been
interested in the way complex organisms interact with viruses and adapt to
them. “With flu
virus, you can watch it change in real time,’’ he said. “You can
watch the antibodies develop
and see when and how it dies out. But with these others you are looking
back tens of
millions of years, so it is hard to know how a virus functioned.’’
Rebuilding of an extinct virus.
While Heidmann was working with the Phoenix virus in France, Bieniasz and
two
colleagues at Aaron Diamond initiated a similar project. (At first,
neither team was aware of
the other’s work.) Bieniasz rebuilt the youngest extinct retrovirus in
the human genome—
one that was still active a few hundred thousand years ago—because it
had the fewest
mutations. The team took ten versions of that virus (we carry more than
thirty) and
compared the thousands of nucleotides in the genetic sequence of each
version. They were
almost identical, but where they differed the researchers selected the
nucleotides that
appeared most frequently. That permitted them to piece together a working
replica of the
extinct retrovirus. “If you have a person with a lethal defect in the
heart,’’ Bieniasz explained,
“and another with a lethal defect in the kidney, you could make one
healthy person by
transplanting the respective organs. That is what we did.
Bieniasz group showed that an extinct retrovirus (HERV-K)
could be resurrected in functional form from molecular fossils that are
present in modern genomes and uncovered evidence of ancient interactions
between APOBEC3 proteins and retroviruses in the form of hypermutated
endogenous proviruses in humans and chimpanzees. They also completed the
first identification of an entry receptor for a presumptively extinct
virus (CERV-2) using a reconstituted ancestral envelope protein.Bieniasz was a 2003
recipient of the Elizabeth Glaser Scientist Award from the Elizabeth
Glaser Pediatric AIDS Foundation and the 2010 recipient of the Eli Lilly
and Company Research Award. He was elected to the American Academy of
Microbiology and received an NIH MERIT award in 2011, and was awarded the
Ohio State University Center for Retrovirus Research Distinguished Career
award in 2015.
Threat from ERV's.
“In the past, you got sick and you keeled over and died.....or you
survived. Nobody
could make much sense of it. But almost ten per cent of our DNA consists
of old
retroviruses, and that says to me that it’s pretty clear they played a
major role in our
evolution. We evolved remarkably sophisticated defenses against them, and
we would have
done that only if their impact on human populations had been quite severe.
It’s very likely
that we have been under threat from retroviruses many times throughout
human history. It is eminently possible that this is not the first time we
have been colonized by a virus very much
like H.I.V.”
Cancer and virus
At the end of the nineteenth century, a mysterious series of cancer
epidemics devastated
American poultry farms. One bird would fall ill and the entire flock would
soon be
dead. In 1909, a desperate farmer from Long Island brought a chicken with
a tumor to the
laboratory of Peyton Rous, a young cancer researcher at the Rockefeller
Institute for Medical
Research, in New York City (which became Rockefeller University). Cancer
was not
supposed to spread by virus, but the bird clearly had cancer. Rous, who as
a young man
worked on a Texas cattle ranch, was mystified. He extracted cancer cells
from the sick bird,
chopped them up, and injected the filtered remains into healthy chickens:
they all developed
tumors. A virus had to be the cause, but for years no one could figure out
how the virus
functioned.
RNA to DNA to injection.
Then, in the nineteen-sixties, Howard Temin, a virologist at the
University of Wisconsin,
began to question the “central dogma” of molecular biology, which
stated that genetic
instructions moved in a single direction, from the basic blueprints
contained within our
DNA to RNA, which translates those blueprints and uses them to build
proteins. He
suggested that the process could essentially run in the other direction:
an RNA tumor virus
could give rise to a DNA copy, which would then insert itself into the
genetic material of a
cell. Temin’s theory was dismissed, like most fundamental departures
from conventional
wisdom. But he never wavered. Finally, in 1970, he and David Baltimore,
who was working
in a separate lab, at the Massachusetts Institute of Technology,
simultaneously discovered
reverse transcriptase, the special enzyme that can do exactly what Temin
predicted: make
DNA from RNA.
Retrovirology
The discovery has had a profound impact on modern medicine. It not only
explained how
cancer can be caused by a virus but provided researchers with the tools
they needed to
understand the origins and natural progression of diseases like AIDS. It
also created a new
field, retrovirology, and, more than that, as the Nobel committee noted
when it awarded the
1975 Prize in Medicine to both Baltimore and Temin, it began to erase the
tenuous borders
between viruses and genes.
ERV's can defend us or attack us.
Retroviruses cause cancers in chickens, sheep, mice, and other animals,
but their effect on
humans became clear only in the late nineteen-seventies, with the
identification of two
viruses that cause forms of leukemia. Retroviral proteins are particularly
abundant in certain
kinds of tumor cells, and scientists wondered to what degree they might be
a cause of cancer.
They were also curious about how retroviruses that infect us today differ
from their
ancestors. Working with mice in 2005, Thierry Heidmann found that
endogenous
retroviruses were present in large quantities in tumor cells. Similar
viruses have been
associated with many cancers (and other diseases). It is still not clear
how they function, but
they may help subvert the immune system, which would permit cancer cells
to grow without
restraint. Endogenous retroviruses also may actually protect us from
viruses that are even
worse, providing intrinsic immunity. Experiments with mice and chickens
have shown that they can block new infections by viruses with a similar
genetic structure. Nonetheless, endogenous retroviruses are parasites, and
in most cases the cells they infect would be better off without them.
There is, however, one notable exception.
Placenta/syncytium
The earliest mammals, ancestors of the spiny anteater and the duck-billed
platypus, laid
eggs. Then, at least a hundred million years ago, embryos, instead of
growing in a shell,
essentially became parasites. While only balls of cells, they began to
implant themselves in
the lining of the womb. The result was the placenta, which permits the
embyros to take
nourishment from the mother’s blood, while preventing immune cells or
bacteria from
entering. The placenta is essentially a modified egg. In the early
nineteen-seventies,
biologists who were scanning baboon placentas with an electron microscope
were surprised to see retroviruses on a layer of tissue known as the
syncytium, which forms the principal barrier between mother and fetus.
They were even more surprised to see that all the animals were healthy.
The same phenomenon was soon observed in mice, cats, guinea pigs, and
humans. For many years, however, embryologists were not quite sure what to
make of these placental discoveries. Most remained focused on the
potential harm a retrovirus could cause, rather than on any possible
benefit. Cell fusion is a fundamental characteristic of the mammalian
placenta but also, it turns out, of endogenous retroviruses. In fact, the
protein syncytin, which causes placental cells to fuse together, employs
the exact mechanism that enables retroviruses to latch on to the cells
they infect.
Viruses and us.
The Nobel Prize-winning biologist Joshua Lederberg once wrote that the
“single biggest
threat to man’s continued dominance on this planet is the virus.”
Harmit Malik, an
evolutionary geneticist at the Fred Hutchinson Cancer Research Center,
acknowledges the
threat, yet he is confident that viruses may also provide one of our
greatest scientific
opportunities. Exploring that fundamental paradox—that our most talented
parasites may
also make us stronger—has become Malik’s passion. “We have been in
an evolutionary arms race with viruses for at least one hundred million
years, there is genetic conflict everywhere. You see it in processes that
you would never suspect; in cell division, for instance, and in the
production of proteins involved in the very essence of maintaining life.
The vif gene.
“One party is winning and the other losing all the time,” Malik went
on. “That’s evolution.
It’s the world’s definitive game of cat and mouse. Viruses evolve, the
host adapts, proteins
change, viruses evade them. It never ends.” The AIDS virus, for example,
has one gene, called “vif,” that does nothing but block a protein
whose sole job is to stop the virus from making copies of itself. It
simply takes that protein into the cellular equivalent of a trash can; if
not for that gene, H.I.V. might have been a trivial disease. “To even
think about the many
million-year processes that caused that sort of evolution,” Malik said.
AIDS
The Hutchinson Center encourages its research scientists to collaborate
with colleagues in
seemingly unrelated fields. Malik and Michael Emerman, a virologist at the
center’s Human
Biology and Basic Sciences Divisions, have been working together for many
years. Malik’s
principal interest is historical: why did evolutionary pressures shape our
defenses against
viruses, and how have they done it? Emerman studies the genetic
composition and molecular
pathology of the AIDS virus. “Together, we are trying to understand what
constellation of
viruses we are susceptible to and why,’’ Emerman said. “We know at
least that it is all a
consequence of infections our ancestors had. So from there we want to try
and derive a
modern repertoire of antiviral genes.”

They focused on chimpanzees, our closest relatives. Chimpanzees are easily
infected by the
AIDS virus, but it never makes them sick. That has remained one of the
most frustrating
mysteries of the epidemic. How did nearly identical genetic relatives
become immune to a
virus that attacks us with such vigor? The most dramatic difference
between the chimp
genome and ours is that chimps have roughly a hundred and thirty copies of
a virus called
Pan troglodytes endogenous retrovirus, which scientists refer to by the
acronym PtERV
(pronounced “pea-terv*”)*. Gorillas have eighty copies. Humans have
none.
“We can see that PtERV infected gorillas and chimps four million years
ago,’’ Emerman told
me. “But there was never any trace of its infecting humans.” It is
possible that all infected
humans died, but it is far more likely that we developed a way to repel
the virus. Nobody
knew why until Emerman, Malik, and Shari Kaiser, a graduate student in
Emerman’s lab,
presented evidence for a startling theory: the evolutionary process that
protects us from
PtERV may be the central reason we are vulnerable to H.I.V.
Pt ERV in chimps, humans have TRIM5 that may kill it..
“We thought we must have a defense against this thing that they don’t
have,’’ Malik said,
picking up the story the following day. Evolutionary biologists are not
given to emotional
outbursts—by definition, they take the long view. Malik is an engaging
and voluble
exception. When an antiviral protein excites him, he doesn’t hold back.
“Where but in
evolutionary history can you see a story like this, with PtERV and the
chimps?’’ he asked,
leaping up from his chair to begin sketching viral particles on a
whiteboard. “It’s simply
amazing.’’ He provided a description of the complex interactions
between viruses and the proteins that we have developed to fight them.
There is one particular gene, called TRIM5a, that in humans manufactures a
protein that may bind to and destroy PtERV.
Trying TRIM5 to kill H.I.V. and Pt ERV.
Like the two human retroviruses that were reconstructed in France and in
New York, PtERV
has long been extinct; Emerman and Malik realized that they would have to
assemble a new
version if they hoped to learn how we became immune to it. They took
scores of viral
sequences and lined them up to see what they had in common. The answer was
almost
everything. When there were differences in the sequence, the researchers
used a statistical
model to predict the most likely original version. Then they put the virus
back together.
(Like Bieniasz, in New York, they did so in such a way that the virus
could reproduce only
once.) They modified the human TRIM5a protein so that it would function
like the chimp
version. After that, the protein no longer protected humans against the
reconstructed copy of
the virus. Next, they tested this modified version against H.I.V. Emerman
placed it in a dish,
first with H.I.V. and next with PtERV. What he found astonished him. No
matter how many
times he repeated the test, the results never varied. “In every case,
the protein blocked either
PtERV or H.I.V.,” Emerman said.
The lentivirus (of which H.I.V. is a a member).
The Oxford University zoology department is housed in a forbidding
concrete structure
that looks like an Eastern European police station. The building is named
for the
Dutch ethologist Niko Tinbergen, whose work—with wasps and gulls, among
other species
—won him a Nobel Prize and helped establish the study of animal behavior
as a science.
Tinbergen’s most famous student, Richard Dawkins, has carried on the
university
tradition of aggressive independence, and so have the younger members of
the faculty. Two members, Aris Katzourakis and Robert Belshaw, both
evolutionary biologists have made the new field of paleovirology a
specialty.
Nobody knows what chain of evolutionary factors is required to transform
an infectious
virus—like H.I.V.—into one that is inherited. Such a virus would have
to invade
reproductive cells. H.I.V. doesn’t do that. It belongs to a class called
lentiviruses (from the
Latin for “slow”), which are common in mammals like sheep and goats.
Because lentiviruseshad never been found in any animal’s genome, most
virologists assumed that they evolved recently. Until this summer, the
oldest known lentivirus was “only” a million years, and almost no one
thought that a lentivirus could become endogenous.
Rabbit virus
In a paper titled “Discovery and Analysis of the First Endogenous
Lentivirus,’’ published
in Proceedings of the National Academy of Sciences, Katzourakis, along
with collaborators
from Oxford, Stanford University, and Imperial College London, showed it
WAS in a rabbit's genome, and thus was ancient and had evolved millions of
years ago. They
discovered the fossilized remains of an ancient lentivirus—the same type
that cause AIDS—within the genome of the European rabbit (Oryctolagus
cuniculus). “At first, I just assumed it was a mistake,’’
Katzourakis said. “We checked it twice, three times. But we kept
seeing genes that are found only in lentiviruses.’’ They named their
discovery “rabbit endogenous lentivirus type K,” or RELIK.
An obvious next step for Katzourakis and his group was to work with
virologists who
assembled a functional version of the ancient virus—as the researchers
in Paris, New York,
and Seattle have done. “It’s the most promising way to explore the
evolution and the impact
of H.I.V.,” he said.
Age of H.I.V.
It might be more than that. AIDS researchers have always been handicapped
by the absence
of a small-animal model in which to study the effects of the disease. It
is not easy to use
monkeys or sheep. They are expensive and difficult to obtain, and, for
reasons of ethics, many experiments on them are proscribed. “Although
RELIK is an ancient lentivirus and only
defective copies were identified in this analysis,’’ the authors
wrote, “recent research has
shown that it is possible to reconstruct infectious progenitors of such
viruses,” which, they
concluded, could potentially “provide a small animal model for
experimental research.”
The discovery has already changed the way scientists think about viral
evolution, and about
H.I.V. in particular. “The most obvious implication is that we can no
longer say that H.I.V.
could not become endogenous,’’ John Coffin, of Tufts, said, though he
still considers that
unlikely. “It opens the field to a whole new level of examination.” It
also considerably alters
the phylogenetic tree. RELIK is at least seven million years old, which
makes it the oldest
known lentivirus. “It is possible that primate lentiviruses such as
H.I.V. and S.I.V.’’—its
simian cousin—“are much older than people ever thought,” Coffin
said.
ERV in koala shows new ERV.
We can’t be certain when endogenous retroviruses entered our genome,
because it is
impossible to watch a five-million-year process unfold. Yet in Australia a
retrovirus seems to
be evolving in front of our eyes. Beginning in the late nineteenth
century, koalas on the
mainland were hunted nearly to extinction. To protect them, as many as
possible were
captured and moved to several islands in the south. In the past hundred
years, those koalas
have been used to replenish the population on the mainland and on several
other Australian
islands. In many cases, though, they have become infected with a
retrovirus that causes
leukemia, immune disorders, and other diseases. It can even kill them. The
epidemic presents a significant threat to the future of the species, and
scientists have followed it closely. One group, from the University of
Queensland, looked for the virus in koala DNA—and, as one would expect
with a retrovirus, found it. The team also noticed that some of the
babies, known as joeys, were infected in the same locations on their DNA
as their parents. That means that the virus has become endogenous. Yet,
when the scientists examined the koalas on Kangaroo Island, in the south,
they discovered something they had not anticipated: none of the koalas
were infected.
Current infection that inserts itself into the genome.
That could mean only one thing: since the infected animals had all been
moved just in the
past century, the koala retrovirus must have spread to Australia recently
and is entering the
genome now. That offers virologists and evolutionary biologists their
first opportunity to
learn how a virus transforms itself from something that can simply infect
(and kill) its host
to an organism that will become a permanent part of that host. Persistent
viruses tend to
grow weaker over the years. They couldn’t live for long if they killed
everything they infected.
How they adapt, though, is a mystery. “Events like this have obviously
occurred in human
evolution,’’ Paul Bieniasz said—even with viruses like H.I.V. “We
might be able to see
how the koala infection settles into the genome, and whether it plays a
role in helping its
host fend off other viruses,” he continued. “Whatever we learn will be
useful, because we
could never have learned it in any other way.
Errors in replication.
The insights provided by recent advances in evolutionary biology have
already been put to
use, particularly in efforts to stop the AIDS virus. One of the main
reasons that endogenous
retroviruses can enter our genome without killing us is that they make
many errors when
they reproduce. Those errors are genetic mutations. The faster a cell
reproduces (and the
older it is), the more errors it is likely to make. And the more errors it
makes the less likely it
is to be dangerous to its host. “Viruses are accumulating and becoming
more decrepit with
every passing million years” was the way Malik described it. That
realization has led
AIDS researchers to contemplate a novel kind of drug. Until recently,
antiviral medications
had been designed largely to prevent H.I.V. from reproducing. Various
drugs try to interfere
with enzymes and other proteins that are essential for the virus to copy
itself. There is a
problem with this approach, however. Because the virus changes so rapidly,
after a while a
drug designed to stop it can lose its effectiveness completely. (That is
why people take
cocktails of H.I.V. medications; the combinations help slow the rate at
which the virus
learns to evade those interventions.)
Forcing mutation.
Scientists at a company called Koronis Pharmaceuticals, just outside
Seattle, are taking the
opposite approach. They hope that by speeding up the life cycle of the
AIDS virus they can
drive it to extinction. The goal is to accelerate the virus’s already
rapid pace of mutation to
the point where it produces such an enormous number of errors in its
genome that it ceases
to pose a threat. Like endogenous retroviruses, H.I.V. would become
extinct. Earlier, researchers at the University of California at San
Francisco and at the University of
Toronto announced an even more fascinating way to use the fossils in our
genome. H.I.V.
infects immune-system cells and alters them so that they can produce more
H.I.V. In doing
so, they stimulate endogenous retroviruses, which then produce proteins
that act as a sort of
distress signal. Those signals can be detected on the surface of H.I.V.-infected
cells, and in
theory it should be possible to develop vaccines that target them. In
essence, such a vaccine
would act like a smart bomb, homing in on a signal transmitted from within
each H.I.V.-
infected cell. The team in San Francisco found strong evidence of those
signals in the
immune cells of fifteen of sixteen volunteers who were infected with H.I.V.
In an uninfected
control group, the signals were far weaker or were absent altogether.
“For a vaccine against an infectious agent, this is a completely new
strategy,’’ Douglas Nixon, the immunologist who led the team, said.
It’s one that could not have emerged without the recent knowledge gained
through experiments with endogenous retroviruses.
Benefits
There may be no biological process more complicated than the relationships
that viruses
have with their hosts. Could it be that their persistence made it possible
for humans to
thrive? Luis P. Villarreal has posed that question many times, most
notably in a 2004 essay,
“Can Viruses Make Us Human?” Villarreal is the director of the Center
for Virus Research
at the University of California at Irvine. “This question will seem
preposterous to most,’’ his
essay begins. “Viruses are molecular genetic parasites and are mostly
recognized for their
ability to induce disease.” Yet he goes on to argue that they also
represent “a major creative
force’’ in our evolution, driving each infected cell to acquire new
and increasingly complex
molecular identities. Villarreal was among the first to propose that
endogenous retroviruses
played a crucial role in the development of the mammalian placenta. He
goes further than
that, though: “Clearly, we have been observing evolution only for a very
short time. Yet we
can witness what current viruses,” such as H.I.V., “can and might do
to the human
population.”
Defective receptors.
There are examples of specific mutations that seem to protect people
against the virus. (For
H.I.V. to infect immune cells, for example, it must normally dock with a
receptor that sits on
the surface of those cells. There are people, though, whose genes instruct
them to build
defective receptors. Those with two copies of that defect, one from each
parent, are resistant
to H.I.V. infection no matter how often they are exposed to the virus.)
The process might
take tens, or even hundreds, of thousands of years, but Darwinian
selection would ultimately
favor such mutations, and provide the opportunity for the evolution of a
fitter human
population. “If this were to be the outcome,’’ Villarreal wrote,
“we would see a new species of
human, marked by its newly acquired endogenous viruses.”
“Viruses may well be the unseen creator that most likely did contribute
to making us
human.”
|